Abstract
Introduction
Response to treatment in patients with multiple myeloma (MM) is variable. With increasing possibilities of treatment regimens, predictive factors for response are important. Immune modulating agents (IMiDs) require Cereblon (CRBN) for activity. Therefore, the aim of this study is to identify the genes of the CRBN pathway which predict the response to therapy with IMiDs. In this abstract the first results are presented.
Methods
Paraffin embedded bone marrow biopsies from patients included in HOVON-87/NMSG-18 trial obtained at inclusion were used for this study. In this trial elderly patients with MM were randomized between treatment with Melphalan-Prednisone (MP)-Thalidomide followed by thalidomide maintenance versus MP-Lenalidomide followed by lenalidomide maintenance (Zweegman et al. Blood 2016;127:1109-1116).
Bone marrow biopsies were stained with a fully automated dual color, bright-field immunohistochemical (IHC) assay for CRBN, its neosubstrates Ikaros and Aiolos and the downstream targets IRF4 and c-MYC. CD138 was used to identify MM plasma cells in the bone marrow samples. For CRBN, both nuclear and cytoplasmic staining was evaluated. The distribution and intensity of the immunostaining was assessed using the H-score. The H-scores were calculated using the following formula: [1 × (% cells 1+) + 2 × (% cells 2+) + 3 × (% cells 3+)] and range from 0-300 (0-600 for combined cytoplasmic-nuclear CRBN H-score). For the Cox regression analysis H-scores were corrected by dividing these by a factor 100: hazard rates were considered per 100 points increase of the H-score. 113 bone marrow samples were selected so far. Protein levels were compared between patients with complete response (CR) or very good partial response (VGPR) vs partial response (PR) and no change (NC). Statistical analysis was done using univariate Cox regression analysis for progression free survival (PFS) and overall survival (OS), and Mann-Whitney test for comparing response groups. Kaplan-Meier survival curves were generated to illustrate survival.
Results
So far, bone marrow samples obtained at diagnosis from 34 patients were evaluated. Fourteen patients were treated in the Thalidomide arm vs 20 patients in the Lenalidomide arm. Median age was 72 years [range 60-84]. ISS stages I/II/III were 38%/47%/15% respectively. At the time of analysis, median follow up of the 16 patients still alive was 53 months [range 18-85 months]. Best response on protocol treatment was sCR in 12%, CR in 9%, VGPR in 35%, PR in 29% and NC in 15%.
Comparing protein expression across the response groups showed a trend of higher levels of combined cytoplasmic and nuclear CRBN in patients who responded better (sCR/CR/VGPR; median H-score: 360 (90-544)) compared to patients with a worse response (PR/NC; median H-score: 273 (185-471)) Mann-Whitney p-value=0.09). In addition, a trend for increased Aiolos expression to be associated with poorer response was found (Mann-Whitney p=0.08; median H-score sCR/CR/VGPR: 251 (162-260) vs PR/NC: 254 (221-267)).
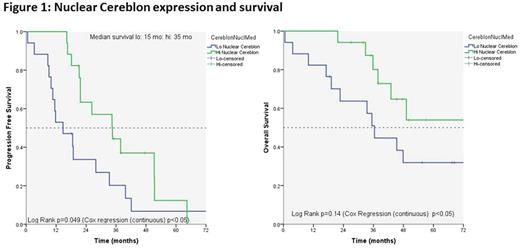
Higher H-score of nuclear staining of CRBN was associated with a longer PFS and OS, with a hazard ratio (HR) of 0.47 for PFS (95% confidence interval (95% CI)=0.24-0.92, p=0.027) and a HR of 0.42 for OS (95% CI=0.19-0.91; p=0.028). Using the median nuclear CRBN expression as an arbitrary cut-off, a significant separation of the lines representing high and low expression could be found for PFS, but not for OS (Figure 1). None of the other markers were associated with survival in the current limited set.
Six patients had high-risk FISH (deletion of 17p and/or translocation t(4;14) and/or translocation t(14;16)) out of 24 patients with known FISH status. These patients did not demonstrate a higher or lower expression of any of the markers evaluated, and showed no association with PFS (log rank p=0.2) but a clear association with shorter OS (log rank p=0.008). As expected, no association between arm and survival was seen.
Conclusions
In this study we demonstrate that higher expression of nuclear CRBN in myeloma cells in bone marrow of patients with MM was associated with a superior PFS and OS. These are early data from an ongoing analysis; a total of 113 bone marrow samples were selected up to now.
Couto: Celgene: Employment, Equity Ownership. Ren: Celgene: Employment, Equity Ownership. Wang: Celgene: Employment, Equity Ownership. Qian: Celgene: Employment, Equity Ownership. Thakurta: Celgene Corporation: Employment, Equity Ownership. Broyl: Amgen: Honoraria; Janssen: Honoraria; Celgene: Honoraria. Zweegman: Amgen: Other: advisory board participation; Takeda: Other: advisory board participation, Research Funding; Janssen: Other: advisory board participation, Research Funding; Celgene: Other: advisory board participation, Research Funding. Sonneveld: Celgene, Amgen, Janssen, Karyopharm, Takeda: Consultancy, Honoraria, Research Funding; Celgene Corporation, Amgen, Janssen, Karyopharm, PharmaMar, SkylineDx: Honoraria; Celgene Corporation, Amgen, Janssen, Karyopharm, SkylineDx, PharmaMar: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal